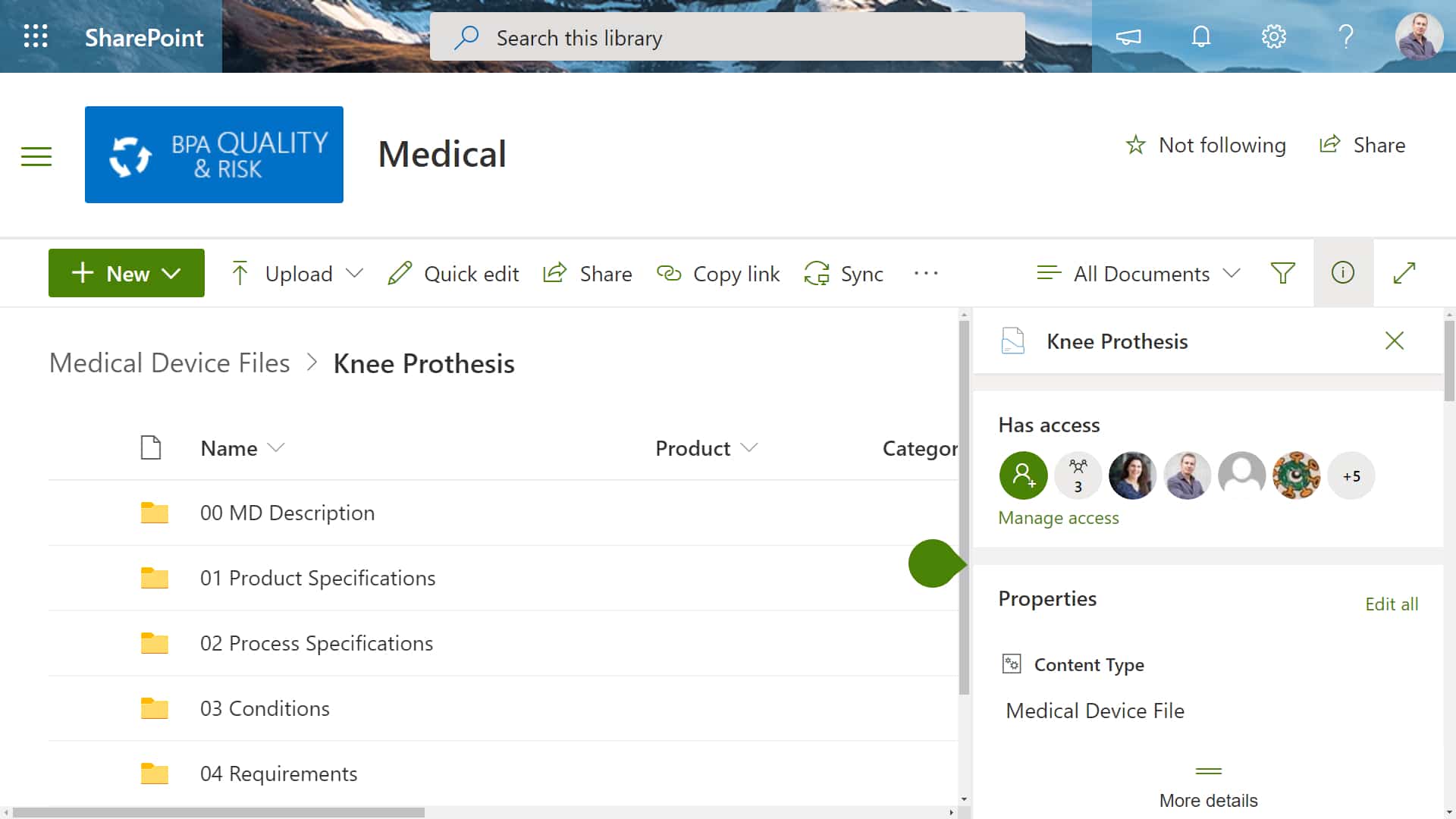
Medical device files are documents that includes descriptions of design records, manufacturing processes, product specifications, device usage guides, quality measurement criteria, levels device files medical of compliance with regulatory bodies and quality standards, and, if required, servicing and installation records and their guidelines. Statistics from the journal of the american medical association state that medical negligence is the third leading cause of death in the country. these shocking statistics highlight the issues around poor treatment and how it can affect bot. A global leader in healthcare it with more than 30 years of experience, our solutions, services and partnership enable organizations worldwide—of all sizes—to change what’s possible in healthcare. Your private medical record is not as private as you may think. here are the people and organizations that can access it and how they use your data. in the united states, most people believe that health insurance portability and accountabil.
See device files medical more videos for medical device files. A person who requires medical care. a person receiving medical or dental care or treatment. a person under a physician's care for a particular disease or condition. a person who is waiting for or undergoing medical treatment and care.
Medical Device Technical File I3cglobal
Jun 28, 2017 medical device files minimize the production time, prevent duplicating processes, and minimize shipment damage within the manufacturing and . Confidential patient medical records are protected by our privacy guidelines. patients or representatives with power of attorney can authorize release of these documents. we continue to monitor covid-19 cases in our area and providers will. An outpatient (or out-patient) is a patient who attends an outpatient clinic with no plan to stay beyond the duration of the visit. even if device files medical the patient will not be formally admitted with a note as an outpatient, their attendance are still registered, and the provider will usually give a note explaining the reason for the visit, tests or procedure/surgery, which should include the names and. emailmap show map share share facebook twitter 7 photos cricket st wigan wn6 7tp tel: 01942 21**** remove_red_eyedisplay wwwemrscouk

Free Medical Records Release Authorization Form Hipaa Word Pdf Eforms Free Fillable Forms

The standard assigns the name "medical device file. " comment: the standard lists the documents that would be included in the medical device file. the content of the medical device file would include the records contained within the device medical record, and additional requirements. The design history file, or dhf, is part of regulation introduced in 1990 when the u. s. congress passed the safe medical devices act, which established new . Nov 19, 2018 what are the differences between design history files (dhf), design history records (dhr) and device master records (dmr). oriel stat a .
Jan 9, 2020 the design history file, or dhf, is part of regulation introduced in 1990 when the u. s. congress passed the safe medical devices act, which . The sub-clause 4. 2. 3 of iso 13485:2016 requires a manufacturer of a medical device to establish a technical file (medical device .
Correct and complete medical records are essential to proper care and billing. start here to learn about gaining access, deciphering codes, and more. thank you, form. email, for signing up. there was an error. please try again. Jan 21, 2020 the core product is a quality manual in microsoft word format, with editable procedures and format documents. the products include all iso .
The add new screen allows you to enter a new listing into your personal medical events record. an official website of the united states government the. gov means it’s official. federal government websites always use a. gov or. mil domain. b. Authorization for release of protected or privileged health information 84182mgh (12/16) mail or fax to: release of information 121 inner belt road, room 240 somerville, ma 02143-4453 phone: 617-726-2361 fax: 617-726-3661 www. massgeneral. org/imaging/about/order_images_films. aspx.
Medical Device File Mdf Per 134852016 4 2 3 Versus Fda

Nov 3, 2019 as mentioned, the technical file is described in annex ii and iii of the mdr 2017/ 745. below i will try to explain to you what is expected in each of . Epic is partnering with tesla to create the world’s first personal mobile telemedicine clinic. the covid-19 pandemic has led device files medical to an explosion of remote care and virtual visit offerings. the goal? keep patients out of healthcare facilities for routine care and advice, leaving hospitals and clinics to focus on more critical needs. Patient care medical is a nationwide catheter supplier & approved medicare provider. we carry all the top brands and make delivery stress-free. 888-726-5066.
Mail Or Fax To Release Of Information 121 Inner Belt Road
Feb 05, 2019 · the difference between epic proficiency vs certification is that with certification, your sponsor pays to send you to epic to take classes before you start studying for exams. with proficiency, you get permission from your employer to study on your own without the cost of travel and the class fees. First, you need to know that the eu mdr 2017/745 is providing a clear view of what should contain a technical file when the mdd 93/42/ec was not so structured.. fortunately, imrdf or ghtf created a template called sted (summary technical documentation medical device) to help organize all the information but this was not mandatory per legislation. Nov 14, 2017 master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound . A library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them.
Whether you're interested in reviewing information doctors have collected about you or you need to verify a specific component of a past treatment, it can be important to gain access to your medical records online. this guide shows you how. The files are documented guidelines which contain a collection of design records, production processes, medical device specifications, product application guidelines, quality acceptance criteria, compliance status of regulatory, quality standards, and, if needed, servicing and installation records, and their guidelines. Asco cancer treatment and survivorship care plansasco developed two types of forms to help people diagnosed with cancer keep track of the treatment they received and medical care they may need in the future: a cancer treatment plan and a su.